Manage Award Overview
- UFIRST – Award Modifications
- Award Terms & Conditions
- Standard NSF Award Terms & Conditions
- Standard NIH Award Terms & Conditions
- NIH Data Management and Sharing
- NASA China Restrictions
- Fiscal Management
- Personnel Management
- Effort Reporting & Management
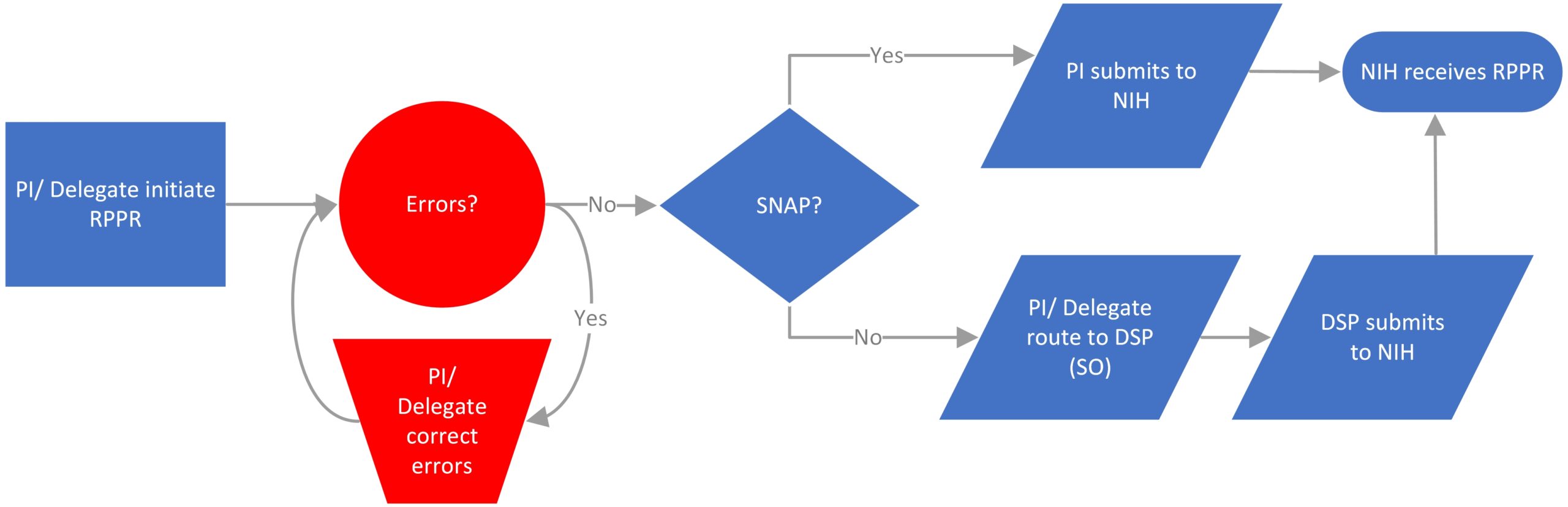
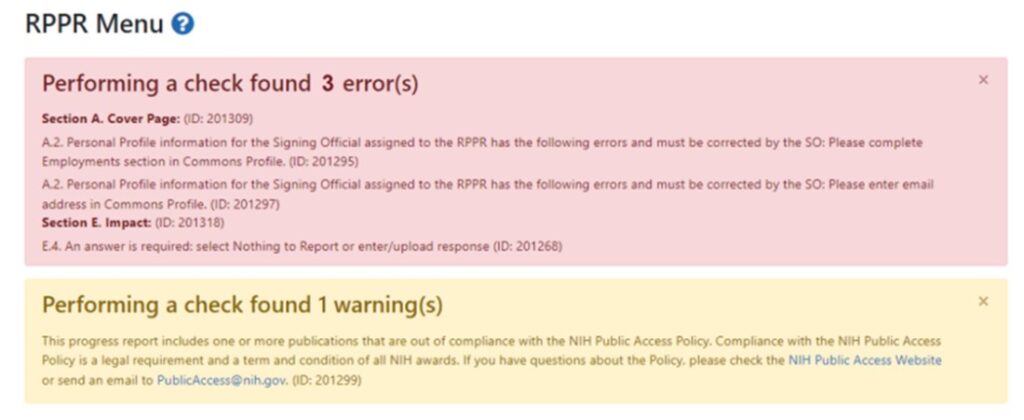
- Progress Reports
- Sponsored Projects Reporting
- Award Closeout
- Federal Agency Grant & Contract Websites
- Frequently Asked Questions