article
by Aaron Hoover downloadable
.pdf
After
decades of being met with blank stares or rolled eyes, veteran
microbiologist Donna Duckworth had almost stopped talking
about an alternative to antibiotics called phage therapy.
Phages
are naturally occurring viruses that attack bacteria. Discovered
more than a century ago, they were a common treatment for
infection before the popularization of antibiotics.
“For
a long time, if you talked about phage therapy, people would
look at you like you were crazy,” says Duckworth, a
professor of molecular genetics and microbiology in the University
of Florida College of Medicine.
But
that has not deterred Duckworth and colleague Paul Gulig from
experimenting with the little-known treatment on a deadly
disease humans can get from eating raw oysters.
Vibrio
vulnificus, a relative of the cholera bacterium that is difficult
to treat with antibiotics, causes dozens of serious illnesses
and a handful of deaths annually. Duckworth and Gulig, also
a professor of molecular genetics and microbiology at UF,
tested Vibrio-attacking phages in diseased mice. The results,
reported last fall in the journal Infection and Immunity,
were impressive.
“It
was very clear that for many of the mice the phage treatment
could completely protect them,” Gulig says.
The
UF research has since evolved into a unique project —
part medicine, part aquaculture — to rid oysters of
the Vibrio bacteria before they reach consumers. The work
is at the forefront of a small but growing body of research
suggesting that phages may offer hope in dealing with bacteria
that are naturally resistant to antibiotics — or have
become resistant due to their widespread use. The latter is
a growing public health problem, with doctors already facing
antibiotic-resistant strains of bacteria behind maladies ranging
from childhood ear infections and pneumonia to sinusitis,
blood infections and meningitis.
“The
reason why people are going back to this is because they are
running out of effective antibiotics,” says Alexander
Sulakvelidze, a University of Maryland microbiologist and
chief scientist at a Maryland biotech start-up that hopes
to become the first to market phages in the United States.
Bacteria
Eaters
The
recent resurgence in interest in phages is only the latest
chapter in the twisted tale of these tiny therapeutic viruses.
By
some accounts, a British chemist named E.H. Rankin first detected
the presence of phages in 1896. The story goes that Rankin
found that water from the River Ganges stopped the spread
of cholera bacteria. However, when the water was boiled, its
powers subsided, suggesting that a living agent was killing
off the cholera.
A
Canadian microbiologist named Felix d’Herelle was among
the first to identify the bacteria-killers as viruses. While
dealing with an epidemic of dysentery among soldiers at a
Paris hospital in 1917, he noticed that some soldiers recovered
on their own. Curious, he found through experiments that the
feces of the recovered soldiers contained a substance that
zapped cultures of the dysentery bacteria. He called this
substance a “bacteriophage” for its ability to
“eat” bacteria, according to Duckworth and Gulig.
d’Herelle’s
publication of his findings touched off a decade-long flurry
of research. In 1926, d’Herelle published a book that
described treating more than a dozen diseases with phages
— including typhoid fever, cholera and bubonic plague.
In the 1930s, Eli Lilly was among the major pharmaceutical
companies that successfully marketed phages. However, when
some commercial phage treatments were reported to be ineffective
and others were blamed for illnesses and deaths, the viruses
began to fall out of favor, Gulig and Duckworth say.
The
1928 discovery of penicillin and subsequent rise of antibiotics
in the West proved to be the death knell for phages, which
quickly faded both from medical research labs and the public
mind. However, due to the relatively high cost of antibiotics,
research and clinical use of phages continued in the East.
Today, phages continue to be sold at pharmacies in Russia,
Poland and the former Soviet Republic of Georgia for intestinal
and wound infections, Duckworth says.
Western
researchers have long used phages for basic science, where
they have been pivotal to most major advances in molecular
biology this century, including the discovery of restriction
enzymes. Researchers found out about these enzymes —
which cut a DNA molecule at specific place and therefore are
essential to recombinant DNA technology — while investigating
why some phages infected some strains of E. coli bacteria
but left others untouched. However, only a handful of researchers,
mostly in Eastern Europe, have probed phages for clinical
uses. Over the past decade or so, that has started to change
as more and more bacteria have become resistant to even the
most potent antibiotics.
The
journal Nature recently reported that more than 40 percent
of the bacteria that cause meningitis, pneumonia, bloodstream
infections, sinusitis and childhood ear infections will be
resistant to the widely used antibiotics penicillin and erythromycin
by next year, up from just 9 percent in 1996. Other evidence
of the pressing need for an antibiotic alternative abounds.
For example, in recent months authorities in Los Angeles and
several other major cities have reported outbreaks of a drug-resistant
form of staph, which causes blood infections in patients in
nursing homes and hospitals, according to news articles.
Phages
and antibiotics kill bacteria in markedly different ways.
Antibiotics kill indiscriminately, poisoning any bacteria
they encounter. Phages are tuned only to a unique host, destroying
its DNA and replacing it with their own, then replicating
and venturing forth to infect more host bacteria.
“Phages
are more like a laser-guided rocket, while antibiotics are
more like an H-bomb,” Sulakvelidze says.
The
difference is at the heart of the promises and pitfalls of
phages for treating disease. On the one hand, their specificity
means they don’t kill off “good” bacteria
such as the ones that naturally break down food in the intestines.
It also means they have none of the side effects associated
with antibiotics. On the other hand, in order for a phage
to work, doctors have to find and administer just the right
one. That means they also have to know the exact bacteria
causing the patient’s infection. While this was difficult
in the early 20th century and contributed to phages’
decline in popularity, modern technology has made identification
easier.
Vibrio
Vulnerability
The
Vibrio vulnificus bacteria occur naturally with microscopic
algae in seawater. When oysters eat the algae, the bacteria
become concentrated. Healthy people who eat these oysters
or are otherwise exposed usually won’t get sick, but
people who suffer from previous liver damage or other conditions
can become seriously ill or die.
Patients
in advanced stages suffer from quarter-sized blood blisters
on their arms and legs. Nationwide, at least 30 people come
down with Vibrio annually. It’s not a public health
crisis, but even one sickness or death can cast a pall over
the oyster industry regionally, shutting down harvesters and
hurting seafood markets and restaurants. Earlier this year,
California banned the sale of all raw oysters harvested from
the Gulf of Mexico from April to October, when the bacteria
are most prevalent.
 |
| The
UF research has since evolved into a unique project —
part medicine, part aquaculture — to rid oysters
of the Vibrio bacteria before they reach consumers. |
“One death is too many for us,” a spokesman for
the California Department of Health Services told the New
Orleans Times-Picayune newspaper.
Antibiotics
are effective in treating Vibrio only if it’s caught
early on. However, the disease is often missed because initial
symptoms resemble the flu.
Seeking
a better treatment, Gulig and Duckworth collected oysters
and mud from the Gulf coast and local seafood markets and
then isolated the naturally occurring phages in the oysters.
They grew the phages in a laboratory and then injected solutions
containing concentrated amounts into mice infected with Vibrio.
The researchers found the phages cured the mice if administered
during a short window of the disease’s progression.
“We
showed that, in typical infections of mice, we get 100 million
bacteria per gram of tissue, and in these treated mice we
could not detect any bacteria at all,” Gulig says.
As
encouraging as the results were, any use of the phages to
cure people suffering from Vibrio — or any other disease
— is years, if not decades, away in the United States.
Among other steps, the phages would have to go through clinical
trials which, as with all new therapies, would be highly controlled
and closely scrutinized by the U.S. Food and Drug Administration
(FDA). Americans seeking cures for infections have journeyed
to Eastern Europe for phage treatment, Gulig says.
So
the project, funded by a $64,000 grant from the U.S. Department
of Commerce Sea Grant Program, is very much the first in a
long series of steps.
But
if follow-up UF research pans out, phages may reach the market
much sooner as sterilizing agents that rid oysters or other
foods of harmful bacteria before they ever reach consumers’
dinner plates. Funded with a second $144,000 grant from Sea
Grant, Gulig and Duckworth are working on the project in collaboration
with Anita Wright, an assistant professor of food science
and human nutrition in UF’s College of Agricultural
and Life Sciences.
An
early stage of the experiment involves intentionally exposing
live oysters to Vibrio bacteria, then introducing phages and
figuring out how many bacteria the virus kills.
Preliminary
results indicate that the phages kill about four-fifths of
the bacteria in the oysters, dropping bacterial counts from
100,000 to 20,000 per gram. That’s about the same as
another experimental technique that uses ultraviolet light
to zap the bacteria in water surrounding the oysters.
“We
would like to knock it down to near zero, since we don’t
know the safe limits for Vibrio,” Gulig says, explaining
that future experiments will probably use “cocktails”
of different phages aimed at attacking many different forms
of the Vibrio bacteria.
If
the researchers can come up with a suitable cocktail, the
next step would be for oyster harvesters, distributors or
seafood markets to “treat” oysters with it —
probably by submerging them briefly in vats of water containing
the phage. There’s no health threat to people because
anyone who eats oysters already eats the natural phage.
Other
researchers have adopted a similar approach but are even closer
to a commercial product. Sulakvelidze is chief scientist at
a start-up called Intralytix, which is developing phages for
sterilizing food-processing plants. He said the Baltimore-based
company is now conducting FDA-approved experimental tests
of a phage that zaps Lysteria monocytogenes, a deadly pathogen,
in a poultry-processing plant. If the results are as good
as lab tests, the company hopes to become the first to market
a phage in the United States.
Says
Duckworth, “I don’t think phages will ever replace
antibiotics, but they will provide one more tool.”
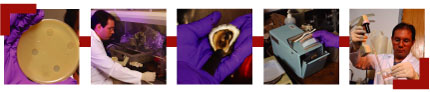 After
growing phages in petri dishes, master's student Julio Martin
introduces them into a tank of oysters. Wearing four gloves
for protection, Martin shucks the oysters, then places them
into an industrial-style blender called a stomacher that beats
them into a brown mush. Martin then uses a pipette to place
samples of the mush into agar growth medium on petri dishes. After
growing phages in petri dishes, master's student Julio Martin
introduces them into a tank of oysters. Wearing four gloves
for protection, Martin shucks the oysters, then places them
into an industrial-style blender called a stomacher that beats
them into a brown mush. Martin then uses a pipette to place
samples of the mush into agar growth medium on petri dishes.
Donna
Duckworth
Professor, Department of Molecular Genetics and Microbiology
(352) 392-0681
duckwort@mgm.ufl.edu
Paul Gulig
Professor, Department of Molecular Genetics and Microbiology
(352) 392-0050
gulig@ufl.edu
Related web site:
http://medinfo.ufl.edu/other/histmed/duckworth/index.html |


