- About
- Research Lifecycle
- Resources
- News & Features
- UF Innovate
Clinical Trials and Clinical Research Projects
On this page:
UFIRST Clinical Research Proposal
UFIRST Clinical Research Award
Clinical Trial F&A Rate
Resources
UFIRST Clinical Research Proposal
All projects with sponsored funding must route through a UFIRST proposal record. It is expected that final budgets for all clinical work are included in the routed proposal. NIH-defined clinical trials and prospective observational clinical research projects will be identified on the UFIRST proposal SmartForm.
- NIH defines a clinical trial as “a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.”
- Prospective observational clinical research is research in which human subjects are identified and then followed forward in time to compare them for a particular health-related biomedical or behavioral outcome, but not assigned to an intervention.
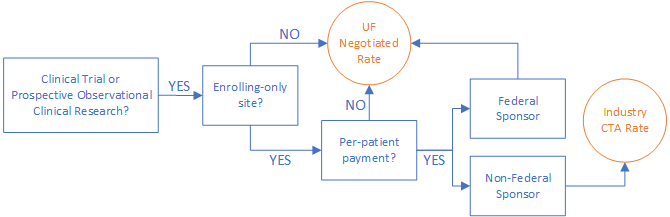
These clinical research projects may enter limited budget information into the UFIRST budget grids if UF is both an enrolling-only site and expects to be paid largely via a per-patient event mechanism.
- To be an enrolling-only site, the Scope of Work for the proposal must meet all of the following requirements: 1) the protocol or research orders are controlled by the sponsor; 2) the UF PI does not have scientific freedom to deviate from the agreed upon protocol or change the experimental design; 3) the protocol follows a clinical calendar which may include visits, data collection, and tissue/sample collection.
- A per-patient event payment mechanism includes payments based on patient enrollment or visits which follow a clinical calendar. Startup, annual, IRB, and closeout fees and invoiceables are allowable in this payment mechanism. Cost reimbursable payments, return of unspent funds, and payments based on set dates or milestones other than per-patient events are not allowable.
If these criteria are met, the only budget information required in UFIRST is completion of the General Budget Information page of the budget SmartForm. No details (effort commitments or cost categories) are required in the budget grids.
UFIRST Clinical Research Award
When setting up a 201 Award for a clinical research project in which UF is an enrolling-only site and is paid largely via a per-patient event mechanism, budget release may be entered by the unit and approved by the appropriate Unit Fiscal Authority (UFA). A TEMP award setup should not be used.
Payments received per patient enrollment/event will be added to the award via UF deposit process. When cash receipts exceed the budget release, the Division of Sponsored Programs Award team will work with the unit to appropriately increase the released amount based on future anticipated enrollment.
Clinical Trial F&A Rate
Resources
UF College of Medicine Clinical Research Hub
Oncore Clinical Trial Management System
Last updated 7/1/25