- About
- Research Lifecycle
- Resources
- News & Features
- UF Innovate
Progress Reports
On this page:
Progress Report Requirements
Progress Report FAQs
NIH Research Performance Progress Reports (RPPR)
NIH RPPR FAQs and Common Errors
ASSIST/Human Subjects System (HSS)
Resources
All sponsors generally require a technical report, so acceptance of sponsored funding will result in the requirement to submit a report(s) at designated times. Principal Investigators (PIs) are responsible for the management and conduct of sponsored activities, including identifying and complying with the technical/programmatic reporting requirements of their awards. PIs should be familiar with reporting due dates, formats, templates, and any online submission portals.
For progress reports that require the signature of an Authorized Official, or submission by an Authorized Official through a sponsor portal, please send the progress report/progress report information and UFIRST award ID to ufawards@ufl.edu and a member of the DSP Award Team will review and reach out to you within 2 business days.
PLEASE NOTE THAT PROGRESS REPORTS CANNOT BE SUBMITTED WITH ERRORS AND THE REVISION PROCESS MAY DELAY TIMELY SUBMISSION.
If you still have questions or concerns after researching how to prepare and submit your reports, please email DSP at ufawards@ufl.edu.
If DSP is notified that a technical report is overdue, DSP will escalate as needed to the respective Department Chair, Associate Dean of Research or Center Director, and Assistant Vice President. Federal regulations may effect corrective actions that have a negative impact on PIs and the University. For example, sponsors may withhold payments to the University or suspend/terminate an award.
Progress Report Requirements
- If your report requires detailing a budget for a future budget period within the award’s competitive segment, use the F&A rate applied at the time of the competitive award. Federal awards require that UF apply an F&A rate for “the life of the award.”
- Even if a report does not require an institutional officer to submit, any statements made to the sponsor and provision of documents that include full and accurate information are binding on the institution. If you have any questions about anything you are submitting such as actual effort expended, budget and balance projections, performance of subrecipient partners, and changes in Current and Pending/Other Support documents or any other binding components, please email ufawards@ufl.edu for assistance.
- Do not communicate financial information. If it is required as part of the form, contact Contracts & Grants Accounting (C&G) for appropriate data to be included.
- Do not disclose unpublished data.
Progress Report FAQs
What should I do if progress report due dates are not listed in the executed award terms and conditions?
Many sponsors do not list technical report due dates in the award document but instead rely on the Funding Opportunity Announcement, Policy Guides, or other resources to communicate progress report due dates. Consult those policy guides, ask your Research Administrator, or reach out to ufawards@ufl.edu if you are unclear on reporting obligations.
What should I do if I cannot meet technical report deadlines?
If you cannot meet technical report deadlines, you should first contact your Program Manager/Technical Contact to see if it is possible to obtain a deadline extension. Be aware that your Program Manager/Technical Contact is not required, or may not have authority, to grant an extension.
Can DSP help me submit my technical reports or obtain due date extensions?
While DSP is happy to help you understand the terms and conditions of your award, including technical report due dates, it is your responsibility as a PI to prepare the technical reports by the due date and obtain technical report due date extensions from your Program Manager/Technical Contact if necessary. If you still have questions or concerns after researching how to prepare and submit your reports, please email DSP at ufawards@ufl.edu.
Who submits financial reports? What about property reports? Invention reports?
Most requests come to ufawards@ufl.edu. While DSP will assist with triaging requests, financial reports are handled by C&G, property reports are handed by Finance & Accounting Department of Asset Management, and Invention Reports are handled by UF Innovate | Tech Licensing.
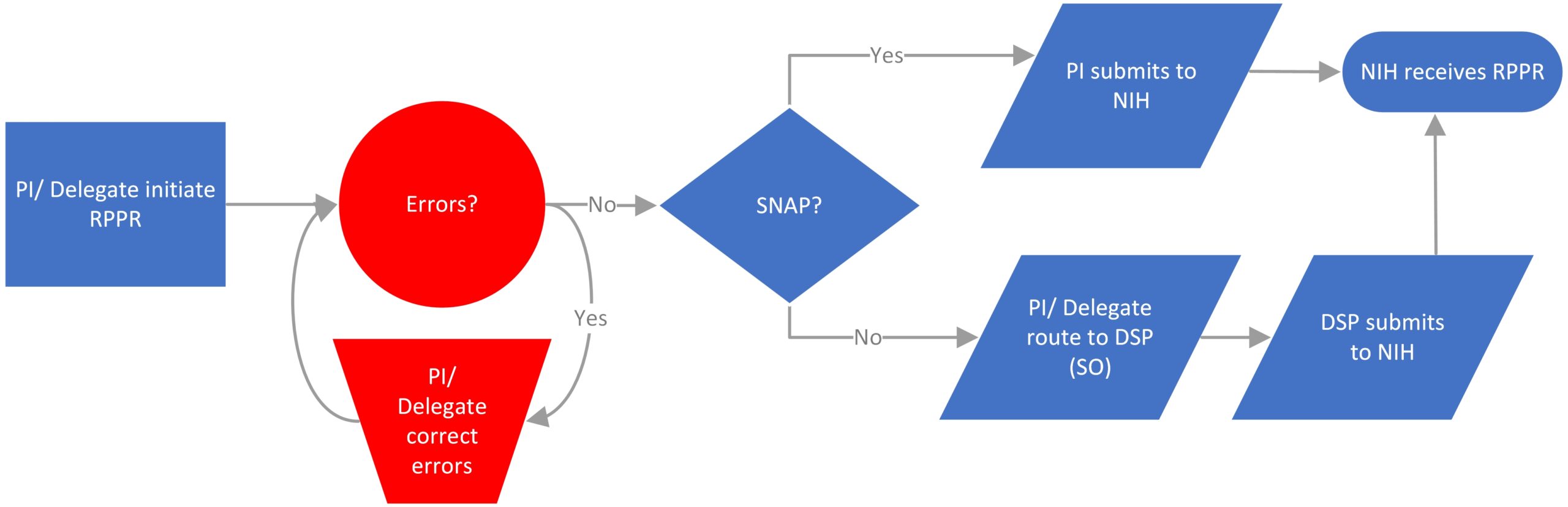
NIH Research Performance Progress Reports (RPPR)
For SNAP awards only, the PD/PI is delegated the ability to submit directly to NIH.
The PI and any delegated assistants (ASST role) can initiate and edit the RPPR. PIs will need to delegate “Progress Report” and “Status” authority to each individual with the ASST role in order for the ASST to access the RPPR. The PI is the only user who will be able to submit SNAP RPPRs directly to NIH.
DSP must submit the RPPR for non-SNAP awards (including the Interim or Final RPPR). All NIH non-SNAP progress reports, which contain detailed budgets, should be routed in eRA Commons to Stephanie Gray no less than 2 days prior to the NIH deadline.
Malign Foreign Talent Recruitment Program (MFTRP) Certification Template: Required for all Senior/Key Personnel annually for RPPRs due on or after January 25, 2026. THIS PDF MUST BE FLATTENED PRIOR TO RPPR SUBMISSION.
| Types of NIH RPPRs | Due Date |
| Interim RPPR: Used when submitting a renewal (Type 2) application. If the Type 2 is not funded, the Interim RPPR will serve as the Final RPPR for the project. If the Type 2 is funded, the Interim RPPR will serve as the annual RPPR for the final year of the previous competitive segment. The data elements collected on the Interim RPPR are the same as for the Final RPPR, including project outcomes. | 120 days from period of performance end date |
| Annual RPPR: Used to describe a grant’s scientific progress, identify significant changes, report on personnel, and describe plans for the subsequent budget period or year. |
SNAP = 45 days before next budget period start date NON-SNAP = 60 days before next budget period start date |
| Final RPPR: Used as part of the grant closeout process to submit project outcomes in addition to the information submitted on the annual RPPR, except budget and plans for the upcoming year. | 120 days from period of performance end date |
Data Management and Sharing Plans in RPPR
Per NOT-OD-24-175, effective for RPPRs due on or after October 1, 2024, a new set of questions has been added asking for details on how recipients are adhering to their approved Data Management and Sharing (DMS) plans, if applicable. The new DMS questions are in section C.5.c and ask:
- Whether data has been generated to date and what type of data it is
- Whether data has been shared for use by others
- If data has been shared, in what repository and under what unique digital identifiers
- If data has NOT been shared, what is the status of data sharing (e.g. being prepared for submission, submitted to repository, not yet expected to be shared); and
- If data has not been generated and/or shared as outlined in the approved DMS plan, what corrective actions have been or will be taken to comply with the approved plan.
Instructions for adding DMS Plan information in the RPPR.
NIH RPPR FAQs and Common Errors
How do I know if an NIH award is SNAP eligible?
The Notice of Award (NOA) Section III – Terms and Conditions should detail if the award was issued under the Streamline Noncompeting Award Process (SNAP).
How does a PI get Submit Authority for SNAP eligible RPPRs?
- If you are a PI affiliated with more than one institution, change institution is selected when you log into Commons using this help guide.
- If you are still unable to submit please email ufawards@ufl.edu to request your delegation.
Why am I unable to edit the RPPR?
Make sure you are the “current reviewer” of the RPPR. If not, select “Recall” which should return the RPPR to you to edit If you are not the Signing Official or PI you will need an Assistant role, requested through this form, and the “Progress Report” and “Status” authorities, delegated by the PI user.
What do I do if I get an error or warning?
Era Commons will not allow RPPR submission if there are errors. A warning will still allow submission. If unable to clear errors, please review the NIH RPPR Common Errors and Warnings and Resources sections of this website. Contact the eRA Help Desk if errors persist.
How do I submit the RPPR to the agency?
If submitting a SNAP RPPR, instead of “Route” use the “Submit” button to submit the report to NIH.
Who should I list as the Signing Official and Administrative Official?
Stephanie Gray
Can other support be signed with a wet signature or typed name?
No, wet and typed names will not be accepted as signatures. Electronic signatures are required. UF recommends DocuSign or Adobe Sign.
There is an error in my RPPR. Can I proceed with submission?
eRA Commons will not allow RPPR submission if there are errors (red text box). A warning (yellow text box) will still allow submission.
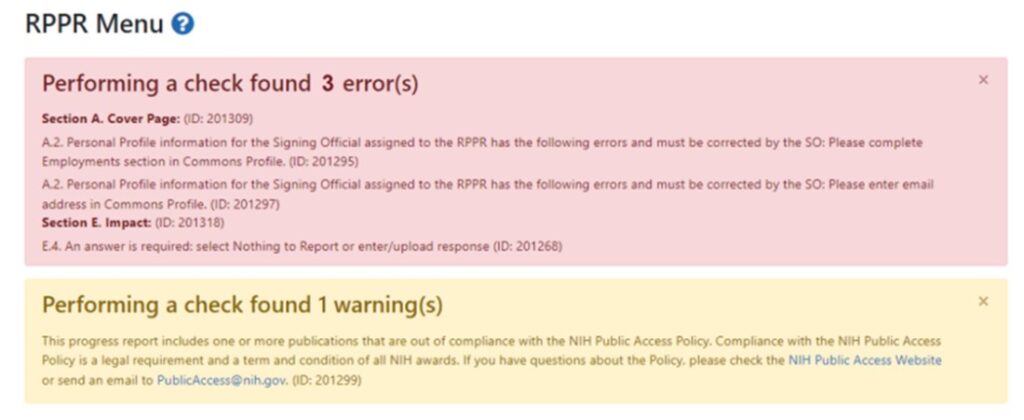
- If you receive an error regarding Section A.2. Personal Profile please ensure the SO and AO selected is Stephanie Gray.
- If you receive an error regarding Section D. Participants:
- Individuals with ‘0’ calendar months of effort should not be listed in this section.
- The PI, as well as those listed on the NOA efforts should be listed.
- Respond to this often overlooked question: Is the individual’s primary affiliation with a foreign organization?
- ALL Undergraduate and/or Graduate Student Roles MUST have a complete eRA Commons account profile, and their Commons User ID must be included in the Participants section before submitting to NIH.
- If the student has left the university, this error can be overridden by changing their role to “other” in section D and manually entering Undergraduate or Graduate student.
- If you receive a warning regarding Section C. Products, please work with your department’s UF library liaison: https://uflib.ufl.edu/specialists/.
- If you receive a warning regarding Section G. Special Reporting Requirements, it is likely because the human subjects data in ASSIST has not been submitted. ASSIST applications must be submitted prior to submitting the RPPR. This includes cumulative enrollment data and inclusion enrollment data. See ASSIST/Human Subjects System (HSS) below.
ASSIST/Human Subjects System (HSS)
The HSS system is a shared system that enables grant recipients to electronically report and update their data on human subjects and clinical trials to NIH; and for NIH agency staff to monitor and manage the data.
Key features:
- Add/update study information;
- Create new enrollment reports or view/edit/update existing enrollment data;
- Make off-cycle corrections or updates after application or Research Performance Progress Report (RPPR) submission;
- Convert a delayed onset study to a full study record, once detailed study information is available;
- Provide interim data as requested by NIH staff, in the funding opportunity announcement, or in the terms and conditions of award;
- Inform NIH of ClinicalTrials.gov registration; and
- Export Human Subjects/Clinical Trial (HSCT) study records in XML format and upload to ClinicalTrials.gov.
Please review NIH’s Overview of Human Subjects System for additional information.
If you have any questions, please contact DSP at ufawards@ufl.edu
ASSIST/Human Subjects System (HSS) FAQs
How do I update my HSS record?
If in a “Submitted” state, the record will need to be reopened to Work in Progress for changes to be made. Note: Revision must be completed BEFORE submitting the RPPR in order for the updated HSS information to appear in the RPPR.
How do I enter my human subject data?
Please refer to the NIH HSS User Guide.
I created an extra Inclusion Enrollment Report (IER) in error. How do I delete?
The PI can delete an inclusion enrollment report (IER) as long as it was not previously-submitted to NIH. Open the study record that contains the extra IER. Click the ‘edit’ button in the Action column of the IER that needs to be deleted, then click the ‘remove report’ button at the button of the screen under the enrollment tables. Click ‘save and release lock’ to save the changes. For existing study records, only the NIH Program Officer can delete.
How do I notify DSP that the ASSIST application is ready to submit?
PIs with delegated submit authority can submit the application to NIH. Otherwise, update the submission status of the application to “Ready to Submit” and notify DSP that your application is ready.
Resources
- NIH RPPR Guide
- HSS User Guide
- eRA Commons help desk | Submit a help desk ticket
- eRA Commons RPPR module online help
- NIH Other Support FAQs
- eRA Commons FAQs
- eRA Commons Training/Help on HSS
Last Updated 02/22/2025