- About
- Research Lifecycle
- Resources
- News & Features
- UF Innovate
NIH Data Management Sharing (DMS) Plan & Repositories
Planning for a project involves making decisions about data resources and potential products. A data management sharing plan describes data that will be acquired or produced during research; how the data will be managed, described, and stored, what standards you will use, and how data will be handled and protected during and after the completion of the project. The Final NIH Policy for Data Management and Sharing is located here: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-013.html.
For assistance with your data management plans, please contact the Strategic Research Development Team.
For assistance with the proposal budgeting process, please review the DMS section of DSP’s Other Direct Costs webpage, or contact the DSP Proposal Team.
Jump ahead to the Repositories.
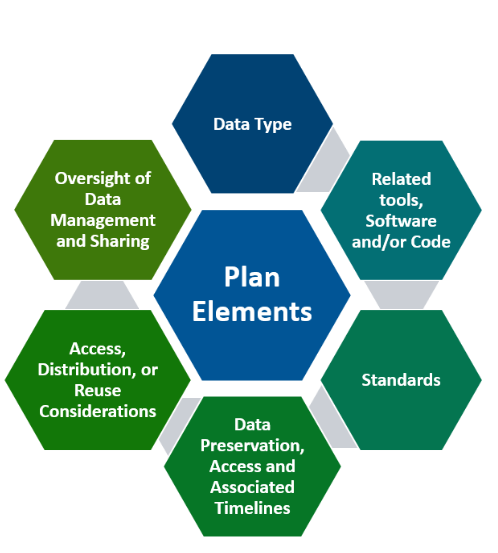
Elements of a Data Management Sharing (DMS) plan
The image of the data management sharing plan provides an overview of how to incorporate FAIR (Findable, Accessible, Interoperable, Reusable) principles while meeting the requirements of the data management sharing plans. To save time and effort, it is important to develop your data management sharing plan at the beginning of your project. Consult with the UF Strategic Research Development team to set a timeline, discuss the new requirements, and determine what campus data services you may need.
- Data type
- Identifying data to be preserved and shared.
- Related tools, software, code
- Tools and software needed to access, manipulate, and analyze data (e.g., code, scripts, hardware, software, and protocols to contribute to greater reproducibility).
- Standards
- Standards to be applied to scientific data and metadata.
- Interoperability means the ways in which data are formatted (i.e., merged or aggregated).
- Data preservation, access, timelines
- Repository to be used (e.g., discipline specific, generalist, institutional).
- Persistent unique identifier, and when/ how long data will be available.
- Access, distribution, reuse considerations
- Description of factors for data access, distribution, controlled (data access request), data privacy, data de-identification, or reuse.
- NLM Data Scrubber
- UF IRB Investigator Guideline on what to include within the Informed Consent process.
- Description of factors for data access, distribution, controlled (data access request), data privacy, data de-identification, or reuse.
- Oversight of data management and sharing
- Plan compliance will be monitored/ managed and by whom.
Data should be shared no later than the time of a publication of findings in a peer-reviewed journal OR at the end of the award, whichever comes first.
Source: NIH
NIH Data Management Sharing Policy
NIH has a longstanding commitment to making the results of NIH-funded funded research available. Responsible data management and sharing has many benefits, including accelerating the pace of biomedical research, enabling validation of research results, and providing accessibility to high-value datasets.
NIH defines scientific data as “the recorded factual material commonly accepted in the scientific community as of sufficient quality to validate and replicate research findings, regardless of whether the data are used to support scholarly publications.”
Scientific data do not include: laboratory notebooks, preliminary analyses, completed case report forms, drafts of scientific papers, plans for future research, peer reviews, communications with colleagues, or physical objects, (e.g., laboratory specimens).
Scientific data will vary depending on the project and the context.
Do you need a DMS Plan?
A complete list of NIH grant programs can be found here. Or, you can use the NIH decision tool to determine what sharing polices apply to your research in 5 minutes or less.
| DMS Policy applies to all research that generates scientific data | DMS Policy does not apply to research and other activities that do not generate scientific data |
| Research Projects | Training (T) |
| Some Career Development Awards (Ks) | Fellowships (F) |
| Small Business SBIR/STTR | Construction (C06) |
| Research Centers | Conference Grants (R13) |
| Resources (Gs) | |
| Research-related Infrastructure Programs (S06) |
Once you determine that your NIH grant requires a DMS plan, follow these five basic steps:
- Review the UF Data Guide (requires Gatorlink authentication) to help you understand your data type within UF’s data classification guidelines.
- Plan & Budget for DMS (Tip: start your DMS planning & budgeting early in the process).
- Submit & Review of DMS Plans (Tip: data plans & budgets are part of the funding proposal; peer review will not see/review DMS plans but will consider related budget items; NIH program staff will review the DMS plan prior to the award).
- Implement the DMS Plan (Tip: manage/share data as describe in the DMS; provide updates on data activities in annual progress reports; work with NIH Program Officer for any changes).
- Attach the DMS plan in the Other Plans section within FORMS-H as part of your NIH applications. FORMS-H must be used for application packages after January 25, 2023. DMS Plans will be collected in a NEW field called “Other Plans” on the PHS 398 Research Plan and Career Development Award forms.
Writing a Data Management & Sharing Plan
This information includes the expectations of NIH, and the required information. Click on the image to download the sample format. Download this DMS checklist to verify you have addressed all the required elements. (Source: Ye, H., et al. (2022), https://doi.org/10.17605/OSF.IO/UADXR). Another option for writing a DMS plan is to use the DMPTool.
Budgeting for Data Management & Sharing Plan
This information includes allowable & unallowable costs, request & justify data management and sharing costs, and an assessment of the budget during the performance periods, including subsequent periods.
NIH recognized that DMS costs may be requested in many cost categories. Applications submitted for due dates on/after October 5, 2023 will no longer require the use of the single DMS cost line item.
NIH will require applicants to specify estimated DMS cost details within the “Budget Justification” attachment of the R&R Budget Form or “Additional Narrative Justification” attachment of the PHS 398 Modular Budget Form, pursuant to the instructions. View the NIH Announcement.
Proposal budgets should include estimated funds needed for data management and sharing activities. These should include all allowable costs for DMS for all data types. Best practice is to plan on full storage costs being incurred during the period of performance. For additional information on budget guidance, please review the UF DSP webpage.
| Allowable costs | Unallowable costs |
| Creating data | Infrastructure costs (F&A) |
| Developing supporting data | Cost for routine conduct of research |
| Formatting data | Costs charged as both direct and indirect |
| De-identifying data | |
| Preparing metadata to support FAIR principles | |
| Local data management considerations | |
| Deposit fees for established repositories |
| Sample | Description | Institute or Center |
| Sample Plan A | Clinical and/or MRI data from human research participants | NIMH |
| Sample Plan B | Genomic data from human research participants | NIMH |
| Sample Plan C | Genomic data from a non-human source | NIMH |
| Sample Plan D | Secondary Data Analysis | NIMH |
You may need to review the best practices for protecting privacy when sharing human research participant data (NOT-OD-22-213):
- De-identify to the greatest extent while maintaining scientific utility; use Common Rule and HIPAA Privacy Rule standards.
- Use agreements for transferring data.
- Understand applicable legal protections and limitations on disclosure.
Be sure to check the NIH Scientific Data Sharing FAQs regularly for updates.
Repositories
As outlined in NIH’s Supplemental Policy Information: Selecting a Repository for Data Resulting from NIH-Supported Research, using a quality data repository generally improves the FAIRness (Findable, Accessible, Interoperable, and Re-usable) of the data. For that reason, NIH strongly encourages the use of established repositories to the extent possible for preserving and sharing scientific data.
When selecting a repository, some programs such as NIH and/or Institute, Center, Office (ICO) policy, and Funding Opportunity Announcements (FOAs) identify a particular repository (or multiple repositories) to be used. Check out the list of NIH-supported scientific data repositories.
Generalist Repositories
NIH supports the Generalist Repository Ecosystem Initiative (GREI) that includes seven established generalist repositories that collaborate to establish consistent metadata, develop use cases for data sharing, train and educate researchers on FAIR data, and the importance of data sharing. These recommended generalist repositories are a great tool for all types of data sets.
Not sure which generalist repository to choose? Download this comparison chart.

- Dataverse: Open source research data repository.
- Dryad: Curated resource that makes research data discoverable, freely reusable, and citable. Dryad provides a general-purpose home for a wide diversity of data types.
- Figshare: Store, share, and discover research to open scientific data to the world.
- Mendeley Data: Free and secure cloud-based communal repository.
- Open Science Framework: Free, open platform to support your research and enable collaboration.
- Vivli: Global clinical research data.
- Zenodo: Open science, open source, FAIR principles, free catch-all repository that assigns DOIs, open & closed data, versioning, GitHub integration, and usage statistics.
Check out the GREI Collaborative Webinar Series on data sharing.
If you feel like exploring more repositories, check out the Registry of Research Data Repositories.
Resources
 You can also use the DMPTool to create your data management. Sign in with your UF GatorLink to create a data management sharing plan from multiple funders.
You can also use the DMPTool to create your data management. Sign in with your UF GatorLink to create a data management sharing plan from multiple funders.- UF Research DSP webpage on NIH Data Management requirements
- UF Libraries Academic Research Consulting & Services
- View the latest presentation on the new NIH DMS Policy (Oct 2022)
- Foreign Collaboration: Updated presentation on expectations
- Check out the NIH Presentation on the DMS Policy to UF (July 2022)
- View the latest recordings for NIH sharing policies.
- Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan
Data sets are scholarly activities.
Citing data is as important as citing any type of publication. Styles differ depending on the citation style but the following information is usually required:
- Author(s): Who is the creator of the data set? Individual, a group, or an organization.
- Year: What year was the data set published? When was it posted online?
- Title of dataset: in italics
- Version: (in parentheses)
- Description: [Data set] or [Unpublished raw data]
- Publisher/distributor: (e.g., database, repository)
- Persistent identifier (e.g., DOI) or Date accessed and URL
Example of cited data sets
APA 7th edition:
Lastname, F. M. or Name of Group (Year). Title of dataset (Version No.) [Data set]. Publisher.
DOI or URL
Use a citation manager to help manage, organize, and store all your citations.
- Free:
- No cost to the UF community, UF VPN required:
Need additional help choosing a citation manager? Reach out to the UF librarians: LIBD-CitationManagers@uflib.ufl.edu